There are many ways to catch invertebrates. Given the wide range of invertebrate types no one method is perfect and different techniques must be used in order to obtain the best picture of the invertebrate fauna as possible. For example, pitfall traps are an efficient method to capture surface moving invertebrates such as spiders. However, since the trap relies on the animals moving and falling into the collecting vessel the trap catch is a combination of abundance AND activity.
A few commonly used techniques are described. For a more complete explanation of these and many other methods see Southwood’s Ecological Methods (2021) Henderson P.A. Fifth Edition, Oxford University Press 528pp. ISBN: 9780198862277
Sweep Net
For flying insects a net is often the easiest method. In areas such as Svalbard it is useful to have a net readily available in order to catch unexpected insects but trapping (see Malaise traps) is often more effective.
Pitfall trap
For groups active on the surface pitfall traps are often surprising effective. Plastic cups with a collecting solution (often water with a little soap) are buried with the cut lip flat with the soil surface. Animals such as spiders and springtails running around will accidently fall into such cups and be trapped. Care must be taken to ensure that the lip of the cup is level with the surface of the ground. This can be challenging if the cup is removed for frequent sampling. A cover over the cup may be appropriate in areas where heavy rainfall may occur or disturbance by other animals, for example grazing, may occur.
Water Traps
Water traps are similar to pitfall traps but consist of a water filled tray placed on the surface. The water film is attractive to flying insects which will often get stuck in the water. Here the animals do not fall in as with pitfall traps but flying insects attracted by the water surface land on the water and due to the detergent fall through the surface.
By using different colours of traps it is possible to target different species groups.
Sticky trap
Sticky traps are commercial traps used to trap pest species in greenhouses. They consist of a plastic sheet covered by a sticky substance. Most sticky traps are either yellow or blue, the colours attract different insect species and are chosen by the farmer in order to catch a particular pest species. While these are effective method to remove pests they are less successful for research since it is often difficult to identify the animals on the trap, hard to remove the animals from the trap even with appropriate solvents, and, not least, everything becomes covered in the trap glue.
Malaise trap
These traps were originally invented by a Swedish entomologist interested in sampling warble flies which parasitise reindeer. They are passive interception traps and work on the simple principle that on encountering an obstacle most insects try and go over rather than around. The trap is a tent-like structure with a central net pegged close to the ground and a sloping roof leading up to the collecting bottle. Insects hitting the vertical net move up the net to the roof, continuing upwards along the roof until they eventually find their way into the collecting bottle filled with a preservative fluid, often ethanol.
Camera trap
Camera traps are also useful for certain studies, for example measuring (abundance and time of visit; ‘phenology’) of the flower visitation of potential pollinator species. Several of these type of traps are active in Björndalen as part of the UNIS BIG project and data analysed as part of masters projects.
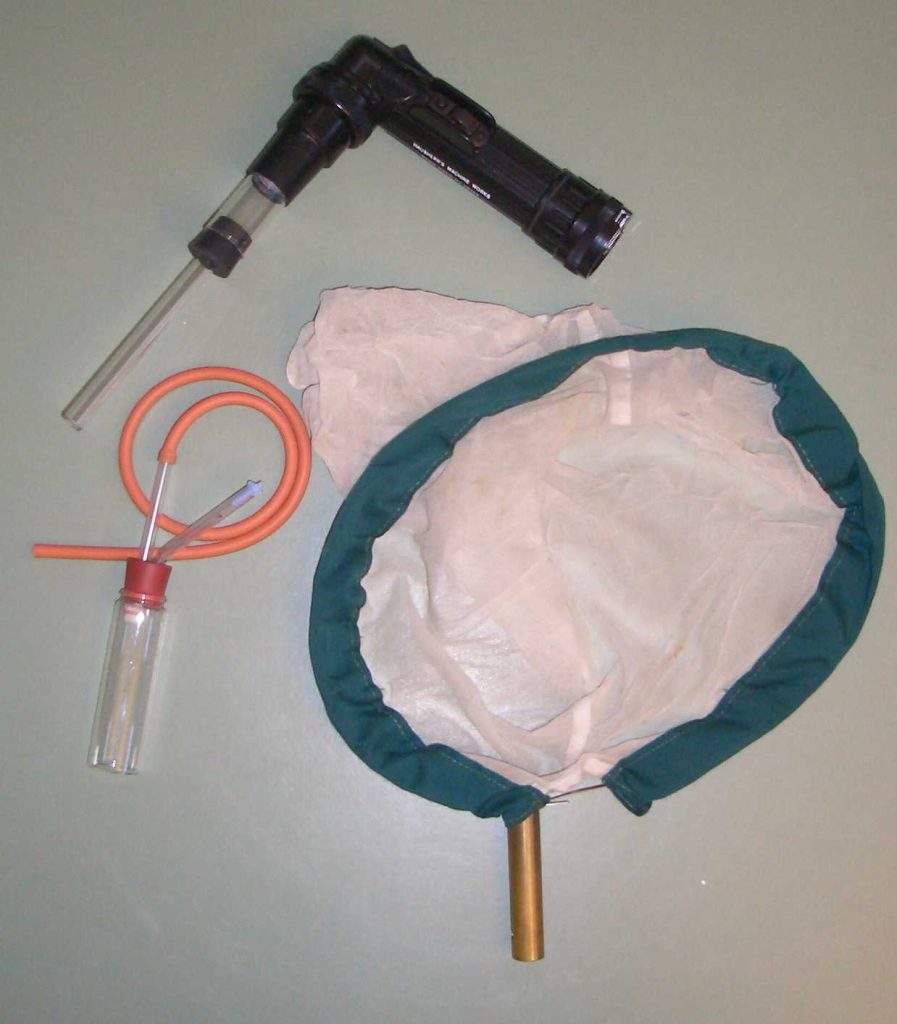
Pooter
Pooters are the entomologists stock tool. The basic concept is that the invertebrates are sucked into the collecting vessel. Three types are commonly available depending on from what substrate the invertebrates are to be collected; either mouth operated, electric, or by squeezy bulb. Most are mouth operated. The advantage of mouth operation is simplicity and fine control of the amount of suction. However, collecting from substrates such as bird’s nests with mouth pooters is obviously unadvisable due to unpleasant fine material being drawn through the gauze into the entomologists mouth. All entomologists are familiar with “pooters-mouth”, a dry mouth caused by the accumulated dust in the mouth following a long successful day pooting. There is also a record of an unfortunate entomologist experiencing the hatching of eggs of Collembola and beetles in his nose after sucking these up by accident (Hurd 1954). For unhealthy substrates, a venturi or electrically powered system is preferable, either commercial or home built.
Soil samples
Many species of invertebrate inhabit the soil. Since the majority are small, under 1 mm in length, it is necessary to remove them from the soil in order to observe them. Several methods exist. The most common is the use of Tullgren funnels or the improved design of MacFadyen (pictures of system – Tullgren and MacFadyen). Although these systems are almost always referred to as extractors it is important to appreciate that they actual expel rather than extract. The animals must be alive in order to leave the soil sample. In the original funnel as described by Tullgren a mesh is fitted inside a funnel on to which the soil sample is placed. The soil sample is always positioned up-side-down in the funnel. A collecting vial is positioned under the funnel and a light bulb over the funnel. The heat of the light bulb progressively dries out the soil. The soil animals start to move downwards away from the drying and warming soil (using the same tunnels and galleyways that they used to get into the soil, hence the reason why the soil is placed up-side-down in the funnel). Eventually, when the soil is completely dried out the animals drop out of the soil and into the collecting vial. The whole process usually takes around several days depending on the size of the soil sample, the power of the light bulb and how wet the soil was to begin with. But how do you know when the core is fully dry? The simplest foolproof method is to tough your cheek with the core surface.If it feels cool you need to continue the extraction a little longer.
This Tullgren funnel is simple and effective but also fails to remove all the animals since many cannot move fast enough and die within the soil. MacFadyen therefore suggested an improved design, the High Gradient. Here the soil is heated gradually over several days with the temperature applied controlled by a thermostat combined with cooling below the cools creating a high temperature gradient. With this system the soil is dried over several days, the temperature being progressively increased as the soil dries. In addition a cooling system is employed to ensure that that the lower side of the soil core is maintained at a low temperature further encouraging the soil animals downwards. Often, the cooling system is switched off for the last period ensuring that the soil core is fully baked dry. The MacFadyen system is considered more efficient than the standard Tullgren although just how much more efficient and for which soil types this applies to is often unknown. However, a major advantage of the MacFadyen system is that it is closed and repeatable. Heating cycles can be pre-programmed and multiple soil samples extracting with the same protocols.
While these techniques are efficient for animals such as mites and Collembola they are less suitable for animals that require a moisture film in which to move, for example the nematodes. Here the O’Conner funnels, a modification of the Tullgren funnel, is often used. The apparatus is effectively a Tullgren funnel but with a tap on the bottom. The tap is closed, soil is positioned in the funnel and water added until the soil is almost, but not entirely, covered. The light is then switched on and the water warmed. The worms then “swim” out of the sample after a few hours and are collected in the water just above the tap. Judicious opening of the tap allows just water with the worms to be removed into a collecting vial.
Light trap
Off great use in many regions is the light trap. Many designs exist but all work around the principle that many species (but not all) night active insects are attracted to lights, especially ultra-violet. These are not often employed on Svalbard for obvious reasons.